
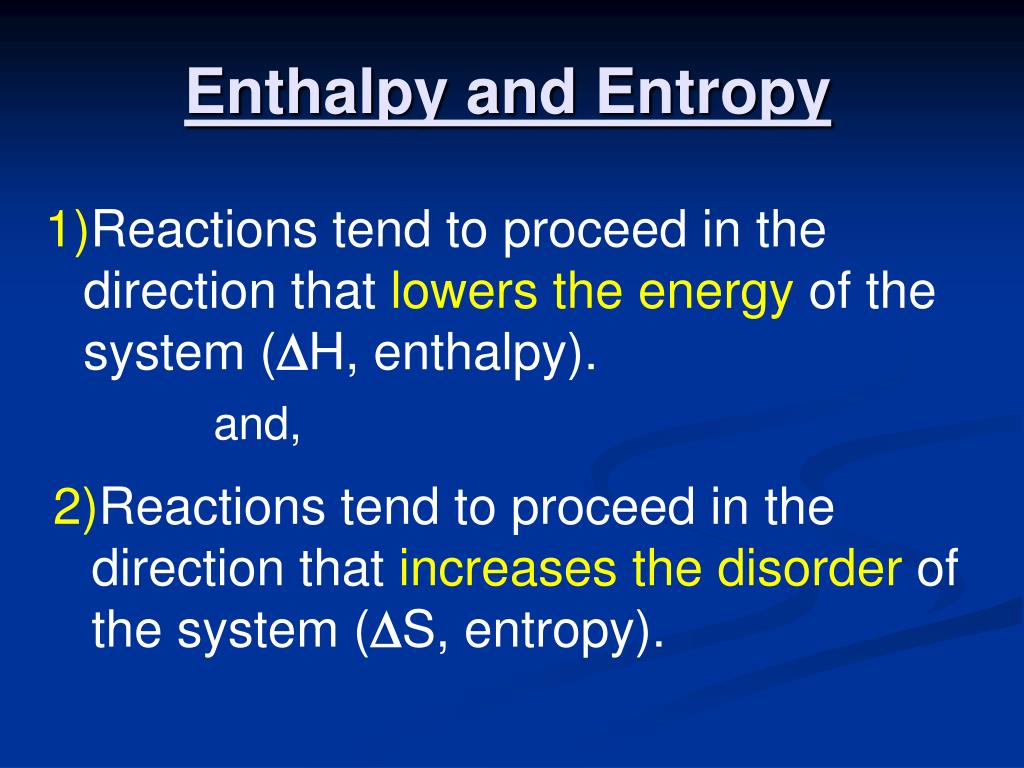
A water molecule that bridges a DNA base with an amino-acid of a protein contributes to enthalpy, but results in loss of entropy, because the system becomes more ordered since the water molecule can no longer move freely. Entropy measures the disorder of the system – the more disordered the solvent and protein-DNA complex are compared to solvent-containing free DNA and protein, the stronger the binding. Enthalpy relates to how strong the chemical bonds that form between the transcription factors and the DNA bases are, compared to a situation where the transcription factor and DNA do not form a complex and bind to water molecules around them. Two physical concepts known as enthalpy and entropy determine the strength of the connection. Transcription factors glide along DNA and bind to short DNA sequences by attaching to the DNA bases directly or through bridges made up of water molecules. Almost all cells carry the same genetic information, but proteins called transcription factors can regulate the activity of genes so that only a relevant subset of genes is switched on at a particular time. Many organisms – including humans – are built of many different types of cells that perform unique roles. The order or sequence of these bases determines the role of a protein.
#Enthalpy vs entropy code
The information in the DNA is stored as a code consisting of four chemical bases, often referred to simply as “A”, “C”, “G” and “T”.

Genes are sections of DNA that carry the instructions needed to build other molecules including all the proteins that the cell needs to fulfill its role.


 0 kommentar(er)
0 kommentar(er)
